BACKGROUND: Frontline treatment with second generation tyrosine-kinase inhibitors (2G-TKIs) in patients with chronic myeloid leukemia in chronic phase (CP-CML) demonstrated higher efficacy as compared to imatinib therapy in inducing deeper molecular responses. However, its ability to reduce treatment failure and progressive disease is debated. Furthermore, limited information is currently available on the outcome of those CML patients showing clinical resistance to first-line treatment with a 2G-TKI.
AIM: To describe the clinical outcome of CP-CML patients showing resistance to a frontline 2G-TKI and that switched to alternative TKIs therapy.
PATIENTS AND METHODS: We performed this investigation on patients drawn from a web-based database (www.epiclin.it/lmc) used to collect clinical and biological data of CML patients enrolled in 24 Italian Hematology Centers from January 2013 onwards. Definitive information about first-line TKI treatment was available in 1277 prospective and consecutive CP-CML patients: 607 received imatinib and 670 dasatinib or nilotinib (2G-TKIs) as frontline therapy. Main inclusion criteria were: i) diagnosis of CP-CML; ii) first-line treatment with a 2G-TKI; iii) switch to second-line treatment in case of clinical resistance (i.e. failure according to ELN 2020 recommendations). Exclusion criteria was switch to second-line treatment for intolerance or for low adherence to therapy. Patients were treated according to the Center policy and the molecular data were obtained in accordance to the IS% parameters.
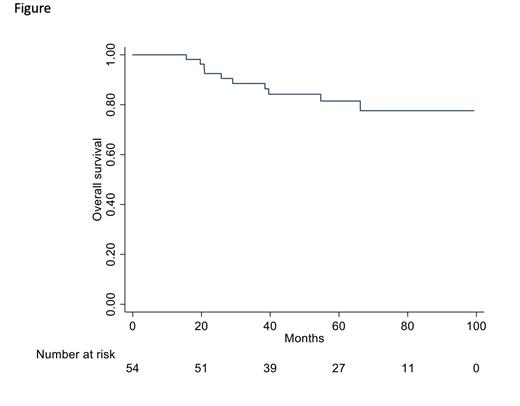
RESULTS: Fifty-four CML patients met the inclusion/exclusion criteria and were identified as failure according to the ELN 2020 recommendations. The median age of these patients was 48.5 (20-81) years; male sex in 30 (55.5%) and female 24 (44.4%), Sokal risk score was low in 16 (29.6%), intermediate in 22 (40.7%) and high in 16 (29.6%) patients respectively;ELTS score was low in 26 (48.1%), intermediate in 14 (25.9%) and high in 14 (25.9%) patients. Additional chromosomal abnormalities were present at diagnosis in 4 out of 54 patients (7.4%: 3 additional Ph-chromosome and 1 trisomy 8). Nilotinib was administered in 27 (50%) and dasatinib in 27 (50%) of the patients.At 6 months, 30 out of 50 evaluable patients (60%) had BCR::ABL1 transcript values >1% and at 12 months 9 of 33 evaluable patients (28%) had BCR::ABL1 values still >1%. Second-line treatment after 2G-TKIs frontline failure started in all patients after a median time of 12 months (3-83) with ponatinib (30 pts, 55.5%), dasatinib (8 pts, 14.8%), nilotinib (4 pts, 7.4%), bosutinib (7 pts, 12.9%) or imatinib (5 pts, 9.2%).At 12 months the switch to second-line treatment induced the achievement of BCR::ABL1 values <1% in 45% of the evaluable cases. Eighteen patients (in which no mutations were found) switched to third-line treatment after a median time of 11 months (0-45) (ponatinib, 8 pts; nilotinib, 1 pt; dasatinib, 3 pts; imatinib, 3 pts; bosutinib, 1 pts; asciminib, 2 pts). MMR or a better response was reached by 6 of these patients and allogeneic stem-cell transplantation (SCT) was performed in 7 patients.Progression to CML advanced phase occurred in 8 pts. After a median follow-up of 59.9 months (15.6 - 99.3) 44 patients were still alive and death occurred in 10 pts (18.5%) (7 CML-related and 3 unrelated). Overall survival (OS) at 5 years was 82% (see Figure below). Indeed, OS at 5 years was 94% for the total 670 patients treated frontline with 2G-TKIs. In this population, 12 out of 32 observed deaths were CML-related and all were recorded in patients who changed TKI therapy, whereas in 19 cases the death was CML-unrelated and in 1 case was not specified. However, those patients who maintained the same therapy for 5 years showed an OS of 96%, whereas those who had to change treatment for any cause showed an OS of 89% at 5 years.
CONCLUSION: Our findings, collected from the prospective cohort of the CML Italian observational registry, show that CP-CML patients failing frontline 2G-TKI therapy, despite a complex management, may have still an acceptable prognosis and survival due to the availability of both multiple TKI options and SCT.
Disclosures
Stagno:Incyte, Novartis, Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Giai:Sobi: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Castagnetti:Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Bonifacio:BMS: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Clinigen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Galimberti:Abbvie, Janssen, Novartis, Roche, Jazz, Astra Zeneca, Pfizer, Incyte: Speakers Bureau. Bocchia:Novartis: Honoraria; Incyte: Honoraria; BMS: Honoraria. Patriarca:Novartis: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Sanofi: Consultancy, Research Funding, Speakers Bureau; Sobi: Consultancy, Speakers Bureau; Alexion: Consultancy. Fozza:Amgen: Research Funding; BMS: Research Funding; Sanofi: Research Funding. Rosti:Novartis /Incyte/Pfizer: Other, Speakers Bureau. Breccia:BMS: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; Novartis: Honoraria; AOP: Honoraria. Pane:Novartis: Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal